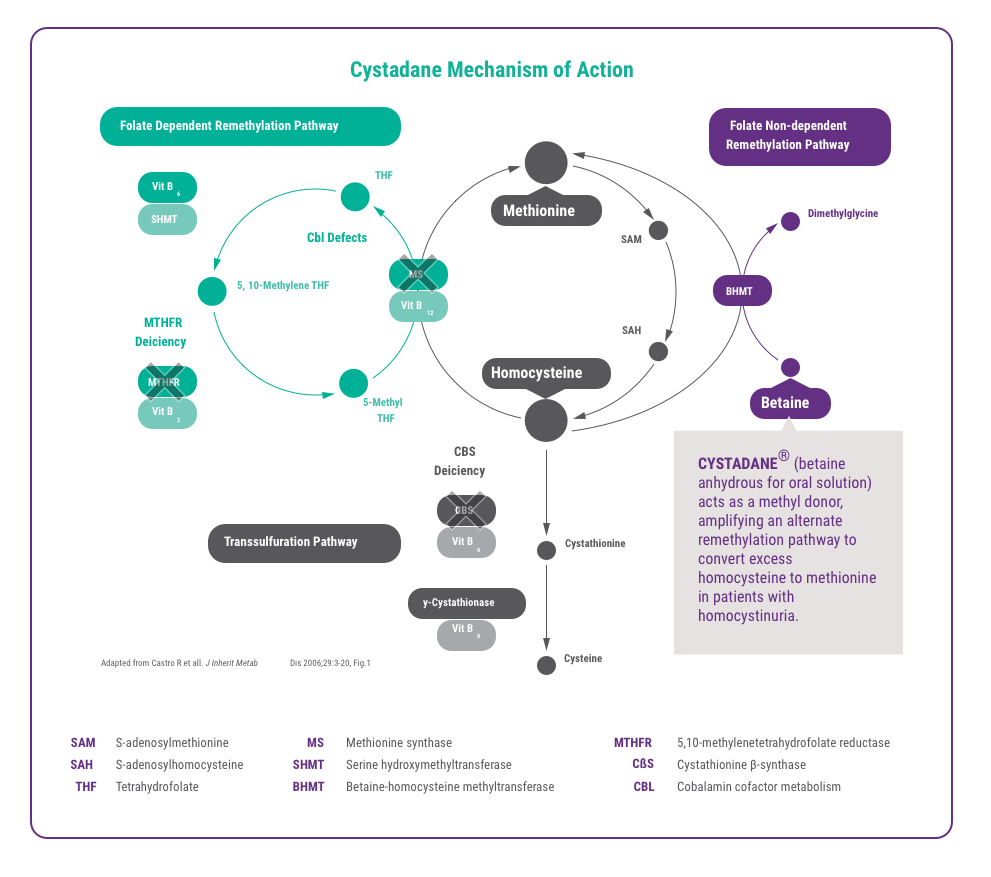
CYSTADANE Provides an Alternate Remethylation Pathway
CYSTADANE acts as a methyl donor, providing an alternate remethylation pathway to convert excess homocysteine to methionine in patients with homocystinuria.1,2,3,4,5
CYSTADANE Mechanism of Action6
CYSTADANE Clinical Data
A meta-analysis of published study data* was performed, which included a double-blind, placebo-controlled, crossover study of 6 patients and 16 additional case studies:
- 78 male and female patients with ages 24 days to 53 years – 48 were diagnosed with CBS deficiency, 13 with MTHFR deficiency, and 11 with cbl defect
- N=62 had numerical plasma homocystine or homocysteine levels reported
- N=48 had clinical response documented7
- N=48 received 6 grams of CYSTADANE per day
- N=57 received CYSTADANE for more than 3 months; 30 patients were treated ≥1 year
*No formal statistical analyses were performed.
Range of percent decrease in plasma concentrations1

98% of patients treated with CYSTADANE showed decreases in either plasma homocysteine or homocystine* concentrations regardless of pre-treatment concentration.7
- Many patients were also taking complementary therapies, such as vitamins B6, B12, and folate.1
- In most cases, adding CYSTADANE further reduced plasma homocystine or homocysteine toward target levels.1,8,9,10,11
*Homocystine is formed nonenzymatically from two molecules of homocysteine1
In about 75% of patients, clinical improvement, such as improvement in seizures or behavioral or cognitive functioning, was reported by treating physicians.1,7
CYSTADANE Safety Profile
CYSTADANE is generally well tolerated.1
Hypermethioninemia and cerebral edema in patients with CBS deficiency were the most serious adverse reactions reported. To keep plasma methionine levels below 1,000 μmol/L, some patients may require dietary modification and, if necessary, a reduction in CYSTADANE dose.1
To determine additional adverse reactions, data was collected through a retrospective survey from 41 physicians who had treated 111 patients with CYSTADANE. Adverse events were retrospectively recalled and were not collected systematically.1
Full physician survey results:
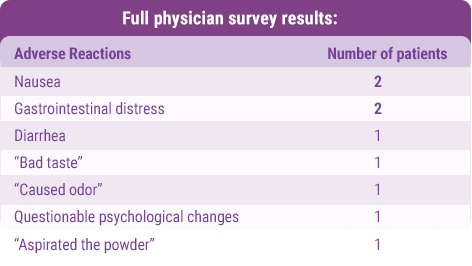
Most common adverse reactions (>2%) were nausea and gastrointestinal distress.1
Pharmacodynamics of CYSTADANE
In patients with low plasma methionine concentrations due to MTHFR deficiency or cbl defect, CYSTADANE has been demonstrated to increase plasma methionine and S-adenosylmethionine (SAM) concentrations.
Patients have taken CYSTADANE for many years without evidence of tolerance. There has been no demonstrated correlation between CYSTADANE concentrations and homocysteine concentrations.
CYSTADANE: Twice daily dosing

Adults and children ≥ 3 years
Recommended dosage: 6 grams per day in divided doses of 3 grams twice daily1

Children <3 years
Recommended starting dosage: 100 mg/kg/day divided in twice daily doses, and then increased weekly by 50 mg/kg increments1
Therapy with CYSTADANE should be directed by physicians knowledgeable in the management of patients with genetic disorders that cause homocystinuria1
- Monitor response to CYSTADANE by plasma homocysteine concentration.1
- Increase dosage gradually until plasma total homocysteine concentration is undetectable or present only in small amounts.1
- Initial response in plasma homocysteine concentrations usually occurs within several days and steady state plasma concentrations occur within a month.1
- Maximum dosage: Dosages of up to 20 grams/day have been necessary to control homocysteine concentrations in some patients. One in vitro simulation study indicated minimal benefit from exceeding 150 mg/kg/day.1

How CYSTADANE is supplied
CYSTADANE is available in plastic bottles containing 180 grams of betaine anhydrous. Each bottle comes with a plastic child-resistant cap and a polypropylene measuring scoop. One level 1.7 mL scoop equals 1 gram of betaine anhydrous powder.1
Administration for Patients
CYSTADANE should only be taken as directed.1
Measure the number of scoops for the patient’s dose with the scoop provided.
One level scoop is equivalent to 1 gram of betaine anhydrous powder.
Mix powder with 4 to 6 ounces of water, juice, milk, or formula until completely dissolved, or mix with food, then ingest mixture immediately.
INDICATIONS AND USAGE
Cystadane is a methylating agent indicated in pediatric and adult patients for the treatment of homocystinuria to decrease elevated homocysteine blood concentrations. Included within the category of homocystinuria are:
- Cystathionine beta-synthase (CBS) deficiency
- 5,10-methylenetetrahydrofolate reductase (MTHFR) deficiency
- Cobalamin cofactor metabolism (cbl) defect
IMPORTANT SAFETY INFORMATION
- Hypermethioninemia in Patients with CBS Deficiency: CYSTADANE may worsen elevated plasma methionine concentrations and cerebral edema has been reported. Monitor plasma methionine concentrations in patients with CBS deficiency. Keep plasma methionine concentrations below 1,000 micromol/L through dietary modification and, if necessary, a reduction of CYSTADANE dosage.
- Most common adverse reactions (> 2%) are: nausea and gastrointestinal distress, based on physician survey.
- To report SUSPECTED ADVERSE REACTIONS, contact Recordati Rare Diseases Inc. at 1-888-575-8344, or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
References
- CYSTADANE Prescribing Information, Recordati Rare Diseases Inc., 2018.
- Kempson SA, Zhou Y, Danbolt NC. The betaine/GABA transporter and betaine: roles in brain, kidney, and liver. Front Physiol. 2014;5:159. doi: 10.3389/fphys.2014.00159
- Kempson SA, Vovor-Dassu K, Day C. Betaine transport in kidney and liver: use of betaine in liver injury. Cell Physiol Biochem. 2013;32(suppl1):32-40. doi: 10.1159/000356622
- Rao TN, Radhakrishna K, Rao TSM, Guruprasad P, Ahmed K. Homocystinuria due to cystathionine beta synthase deficiency. Indian J Dermatol Venereol Leprol. 2008;74(4):375-378.
- Sacharow SJ, Picker JD, Levy HL. Homocystinuria caused by cystathionine beta-synthase deficiency. 2004 Jan 15 [Updated 2017 May 18]. In: Adam MP, Ardinger HH, Pagon RA, et al., editors. GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle; 1993-2018. Available at: https://www.ncbi.nlm.nih.gov/books/NBK1524/.
- Castro R, Rivera I, Blom HJ, Jakobs C, de Almeida IT. Homocysteine metabolism, hyperhomocysteinaemia and vascular disease: An overview. J Inherit Metab Dis. 2006;29:3-20.
- Data on File, Recordati Rare Diseases Inc.
- Morris AAM, Kozich V, Santra S, Andria G et al. Guidelines for the diagnosis and management of cystathionine beta-synthase deficiency. J Inherit Metab Dis. 2017;40(1):49-74. doi: 10.1007/s10545-016-9979-0
- Schiff M, Blom HJ. Treatment of inherited homocystinurias. Neuropediatrics. 2012;43(6):295-304. doi: 10.1055/s-0032-1329883
- Sloan JL, Carrillo N, Adams D, Venditti CP. Disorders of intracellular cobalamin metabolism. 2008 Feb 25 [Updated 2018 Sep 6]. In: Adam MP, Ardinger HH, Pagon RA, et al., editors. GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle; 1993-2018. Available at: https://www.ncbi.nlm.nih.gov/books/NBK1328/.
- Huemer M, Diodato D, Schwahn B, Schiff M et al. Guidelines for the diagnosis and management of the cobalamin-related remethylation disorders cblC, cblD, cblE, cblF, cblG, cblJ and MTHFR deficiency.J Inherit Metab Dis. 2017;40:21-48.
IMPORTANT SAFETY INFORMATION
- Hypermethioninemia in Patients with CBS Deficiency: CYSTADANE may worsen elevated plasma methionine concentrations and cerebral edema has been reported. Monitor plasma methionine concentrations in patients with CBS deficiency. Keep plasma methionine concentrations below 1,000 micromol/L through dietary modification and, if necessary, a reduction of CYSTADANE dosage.
- Most common adverse reactions (> 2%) are: nausea and gastrointestinal distress, based on physician survey.